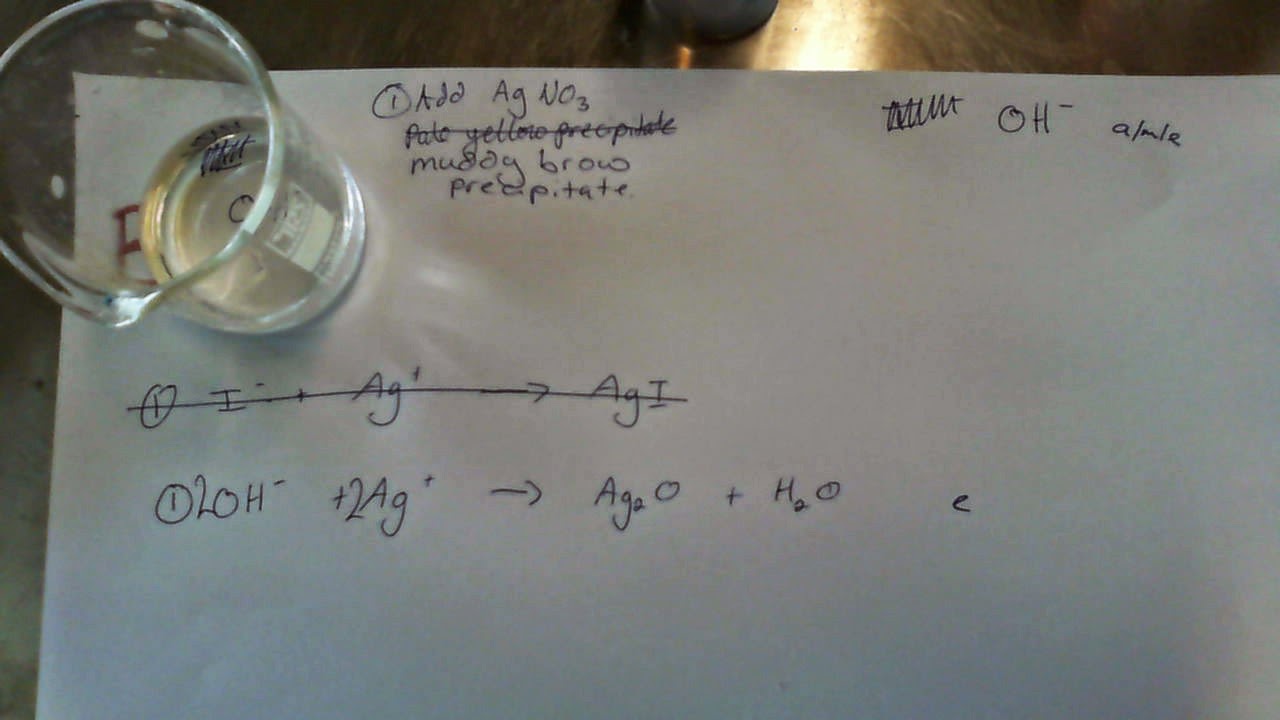Anions are negative ions. We only need to learn about how to identify six of them, and the flow chart is given to us. None of these ions are coloured, so we rely on precipitation, neutralisation and complex ion formation reactions instead:
Silver ions will make a pale-coloured precipitate with both Chloride and Iodide ions. The difference here is that Silver Chloride will form the Silver Diammine Complex Ion with excess Ammonia solution, whereas Silver Iodide will not. Therefore, the final equation is the reaction between the Silver ions and Ammonia (not the Chloride ions).
Silver ions make a muddy brown precipitate with Hydroxide ions; this is a much darker colour than the precipitate formed with Chloride or Iodide ions. Again, keep in mind that the precipitate is Silver Oxide as Silver Hydroxide does not exist.
Confirm that it is Iodide (not Chloride) by trying to redissolve the precipitate with excess Ammonia solution.
Be careful to balance the ions on both sides of the equation.
The last equation is considered evidence of Excellence-level thinking, as it is not a precipitation equation.
We did not bother with Nitrate, as there are no reactions (so no equations) for this ion.
Ions In Solution
Wednesday, 24 September 2014
Cation Experiments
Today, Dominique worked through identifying some unknowns. Here are her results, along with some notes from errors made, or questions asked during this activity.
The third step was done just to confirm that it wasn't Barium.
While Ammonium is a possible cation, it is not going to be included in the assessment. The Achievement Standard criteria state that candidates will not be expected to distinguish between Sodium and Ammonium ions.
Although it is blatantly obvious that this is a Copper (II) solution, we must confirm it. There is no point adding NaOH, as the same thing can be achieved by adding a few drops of Ammonia solution (Ammonia is a base, so also provides Hydroxide Ions).
Examiners love using Zinc, Aluminium and Lead as unknown cations - just look at all of the wonderful complex ions!! Note the charge of the complex ion. Zn = 2+ and each OH = -, therefore the net charge is 2- (+2 + -4).
The addition of NaOH makes it obvious that this is is Iron (III). However, if you shake a solution of Iron (II) Hydroxide, it will oxidise the Iron (II) into Iron (III). Therefore, testing a new sample with KSCN is a vital step to confirm it is Iron (III), not Iron (II).
Barium is actually one of the easiest ones to identify, so long as you remember that it might form a white precipitate with NaOH.
Be very careful not to shake the test tube upon the formation of the green precipitate; this will lead to the Iron (II) being oxidised to Iron (III), making your results confusing.
While the underlined equation is accepted, it is incorrect; there is no such thing as Silver Hydroxide. If you can remember the correct equation for the precipitation of Silver Oxide (Silver ions + Hydroxide ions), then this counts towards Excellence.
The Silver Diammine complex ion equation is also an Excellence opportunity, and one that doesn't follow the "if you're unsure, it is four" rhyme/rule.
Aluminium is another one with a lovely complex ion. It does obey the "if you're unsure, it is four" rhyme . Note that this gives the complex ion a -1 charge.
This one was used to show the amount of detail required. The ions identified on the right-hand side are the possible ions after each test. Barium must be considered as a possible ion when a white precipitate is formed with NaOH due to the concentrations of solutions that we are using. Barium hydroxide is a sparingly-soluble compound, so may precipitate despite what our flow chart says.
The third step was done just to confirm that it wasn't Barium.
While Ammonium is a possible cation, it is not going to be included in the assessment. The Achievement Standard criteria state that candidates will not be expected to distinguish between Sodium and Ammonium ions.
Although it is blatantly obvious that this is a Copper (II) solution, we must confirm it. There is no point adding NaOH, as the same thing can be achieved by adding a few drops of Ammonia solution (Ammonia is a base, so also provides Hydroxide Ions).
Examiners love using Zinc, Aluminium and Lead as unknown cations - just look at all of the wonderful complex ions!! Note the charge of the complex ion. Zn = 2+ and each OH = -, therefore the net charge is 2- (+2 + -4).
The addition of NaOH makes it obvious that this is is Iron (III). However, if you shake a solution of Iron (II) Hydroxide, it will oxidise the Iron (II) into Iron (III). Therefore, testing a new sample with KSCN is a vital step to confirm it is Iron (III), not Iron (II).
Barium is actually one of the easiest ones to identify, so long as you remember that it might form a white precipitate with NaOH.
Be very careful not to shake the test tube upon the formation of the green precipitate; this will lead to the Iron (II) being oxidised to Iron (III), making your results confusing.
While the underlined equation is accepted, it is incorrect; there is no such thing as Silver Hydroxide. If you can remember the correct equation for the precipitation of Silver Oxide (Silver ions + Hydroxide ions), then this counts towards Excellence.
The Silver Diammine complex ion equation is also an Excellence opportunity, and one that doesn't follow the "if you're unsure, it is four" rhyme/rule.
Aluminium is another one with a lovely complex ion. It does obey the "if you're unsure, it is four" rhyme . Note that this gives the complex ion a -1 charge.
Tuesday, 9 September 2014
Colourless Cations
Most of the cations we need to identify are colourless: Ag+, Mg2+, Pb2+, Ba2+, Zn2+, Al3+, Na+ and NH4+.
This makes them a bit tougher to identify in an unknown solution.
Muddy Brown Precipitate = Ag+
No Precipiatate = Na+ or NH4+ (you will not be asked to tell these two ions apart in an assessment)
White Precipitate = any of the others
Precipitate Remains = Ba2+ or Mg2+
Precipitate Disappears (redissolves) = Pb2+, Zn2+or Al3+
Collect a new sample if it is not Pb2+
Add excess/concentrated ammonia. Both will form a white precipitate with the hydroxide ions in ammonia (it is a base), but zinc will redissolve, forming a complex ion with the ammonia molecules. Aluminium will not make this complex.
No Precipitate = Zn2+
White Precipitate = Al3+
This makes them a bit tougher to identify in an unknown solution.
Step One
The easiest way to start is to add a few drops of NaOH:Muddy Brown Precipitate = Ag+
No Precipiatate = Na+ or NH4+ (you will not be asked to tell these two ions apart in an assessment)
White Precipitate = any of the others
Step Two
If a white precipitate has formed, add another 2-3 mL of NaOH:Precipitate Remains = Ba2+ or Mg2+
Precipitate Disappears (redissolves) = Pb2+, Zn2+or Al3+
Barium or Magnesium?
Collect a new sample and add about 1 mL of sulfuric acid. The sulfate ions will form a white precipitate with barium ions, but not with magnesium:
White Precipitate = Ba2+
No Precipitate = Mg2+
Lead, Zinc or Aluminium?
Collect a new sample and add a few drops of potassium iodide (KI):
Yellow Precipitate = Pb2+
No Precipitate = Al3+or Zn2+
Add excess/concentrated ammonia. Both will form a white precipitate with the hydroxide ions in ammonia (it is a base), but zinc will redissolve, forming a complex ion with the ammonia molecules. Aluminium will not make this complex.
No Precipitate = Zn2+
White Precipitate = Al3+
Identifying Coloured Cations
Three of the cations we work with have characteristic colours, although iron (II) can often be very pale.
Cu2+ = blue
Fe2+ = pale green
Fe3+ = orange
While this is very useful for deciding which ion might be present in an unknown solution, but we also need some chemical reaction to confirm which cation is present.
One of the easiest tests to do is the addition of a few drops of NaOH:
Cu2+ + 2OH- → Cu(OH)2 (pale blue precipitate)
Fe2+ + 2OH- → Fe(OH)2 (forest green precipitate)
Fe3+ + 3OH- → Fe(OH)3 (orange/rust precipitate)
The only issue with these (on their own) is that the Fe2+ reaction often has an orange/rust coloured precipitate develop on top if it is left for a while. Therefore, we need some more definite experiments:
Fe3+ + SCN- ↔ [FeSCN]2+
Fe2+ will not have this effect; in fact, there will be no reaction at all.
Cu2+ = blue
Fe2+ = pale green
Fe3+ = orange
While this is very useful for deciding which ion might be present in an unknown solution, but we also need some chemical reaction to confirm which cation is present.
NOTE: There is an error in the video regarding the copper-ammonia complex ion. This is corrected in the text below.
One of the easiest tests to do is the addition of a few drops of NaOH:
Cu2+ + 2OH- → Cu(OH)2 (pale blue precipitate)
Fe2+ + 2OH- → Fe(OH)2 (forest green precipitate)
Fe3+ + 3OH- → Fe(OH)3 (orange/rust precipitate)
The only issue with these (on their own) is that the Fe2+ reaction often has an orange/rust coloured precipitate develop on top if it is left for a while. Therefore, we need some more definite experiments:
Iron (III)
If you need to confirm that the orange precipitate was definitely due to Fe3+, get a new sample and add a few drops of potassium thiocyanate (KSCN). This goes blood-red, due to a complex ion being formed:
Fe2+ will not have this effect; in fact, there will be no reaction at all.
Copper (II)
It seems a bit pointless to confirm the presence of Cu2+ as its blue colour is obvious, and the precipitate it forms with NaOH is unique. However, we are still expected to do the following experiment as well:
A new sample is collected, and excess (or concentrated) ammonia is added. It goes from a pale blue solution to a pale blue precipitate, then very quickly develops into a royal blue solution. This is another complex ion:
Cu2+ + 4NH3 ↔ [Cu(NH3)4]2+
Please note that this is a correction of the equation shown in the video.
As Fe2+ is a very pale green colour, it can be confused for Cu2+ initially. If ammonia is added to Fe2+, it will make a forest green precipitate due to the hydroxide ions in the ammonia (ammonia is a base).
A new sample is collected, and excess (or concentrated) ammonia is added. It goes from a pale blue solution to a pale blue precipitate, then very quickly develops into a royal blue solution. This is another complex ion:
Cu2+ + 4NH3 ↔ [Cu(NH3)4]2+
Please note that this is a correction of the equation shown in the video.
As Fe2+ is a very pale green colour, it can be confused for Cu2+ initially. If ammonia is added to Fe2+, it will make a forest green precipitate due to the hydroxide ions in the ammonia (ammonia is a base).
Subscribe to:
Comments (Atom)