Anions are negative ions. We only need to learn about how to identify six of them, and the flow chart is given to us. None of these ions are coloured, so we rely on precipitation, neutralisation and complex ion formation reactions instead:
Silver ions will make a pale-coloured precipitate with both Chloride and Iodide ions. The difference here is that Silver Chloride will form the Silver Diammine Complex Ion with excess Ammonia solution, whereas Silver Iodide will not. Therefore, the final equation is the reaction between the Silver ions and Ammonia (not the Chloride ions).
Silver ions make a muddy brown precipitate with Hydroxide ions; this is a much darker colour than the precipitate formed with Chloride or Iodide ions. Again, keep in mind that the precipitate is Silver Oxide as Silver Hydroxide does not exist.
Confirm that it is Iodide (not Chloride) by trying to redissolve the precipitate with excess Ammonia solution.
Be careful to balance the ions on both sides of the equation.
The last equation is considered evidence of Excellence-level thinking, as it is not a precipitation equation.
We did not bother with Nitrate, as there are no reactions (so no equations) for this ion.

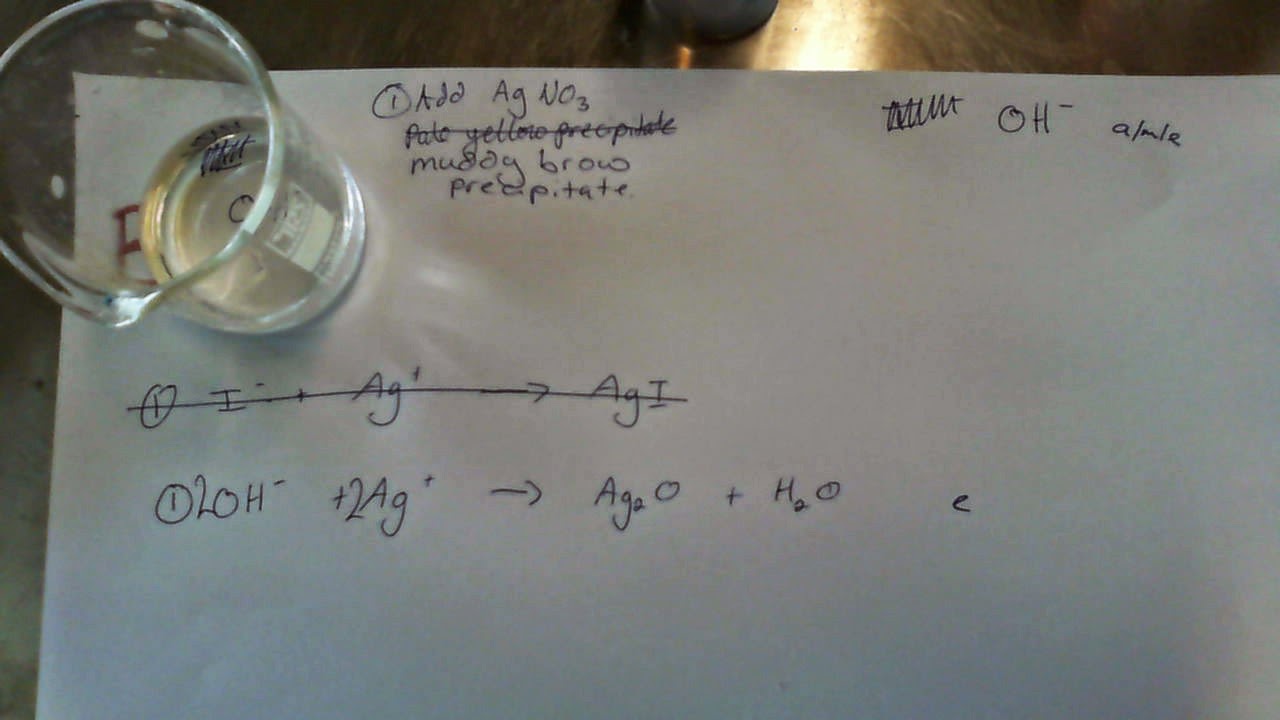



No comments:
Post a Comment